
PRODUCT INSIGHTS
TWO VERSIONS FOR THE TREATMENT OF MALE STRESS URINARY INCONTINENCE
ARGUS is an adjustable suburethral system designed to treat male stress urinary incontinence due to intrinsic sphincter deficiency1. It consists of a central silicone foam pad, two arms with conical segments and two washers to fix the system. No meshes present in its design2. ARGUS offers two different versions, retropubic and transobturator, to choose the best approach for each indication and patient condition. Both options contain a complete and easy-to-use surgical kit that facilitates a safe and minimally invasive procedure2.
5 YEARS WARRANTY


- ARGUS Instruction for use
- Promedon files
PRODUCT HIGHLIGHTS
SUBURETHRAL PAD
The compressible suburethral PAD, composed of radiopaque silicone foam, has been designed to allow coaptation of the bulbar urethra, generating upward pressure when pulling the silicon arms.
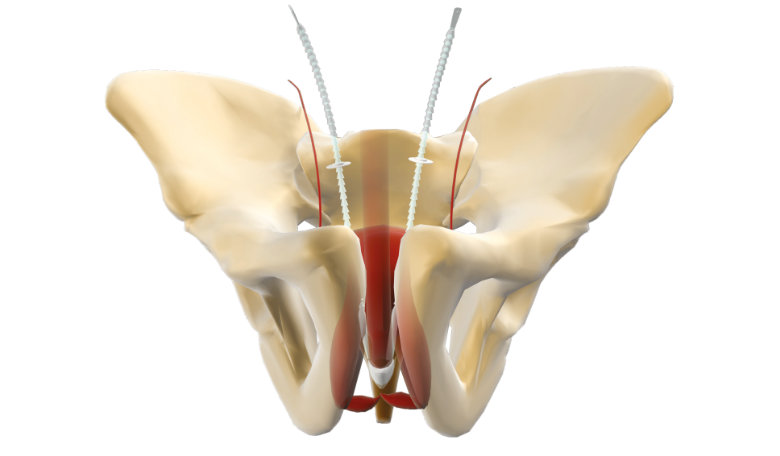
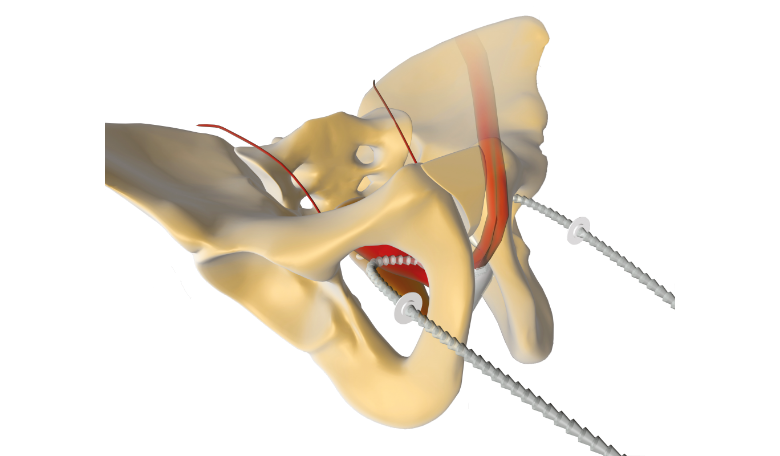

ADJUSTABLE FIXATION SYSTEM
ARGUS was developed with a unique intraoperative fixation mechanism. The Ring positioners (washers) are inserted in both silicone arms to create the fixation point against the muscle wall.
ADJUSTABILITY: Postoperative tension adjustments can be performed according to the needs and conditions of each individual patient with a minimally invasive method


ARGUS NEEDLES
ARGUS needles have been designed for a safe insertion and deployment of the implant fixation arms. The curve of both type of needles has been designed to fit the male anatomy.
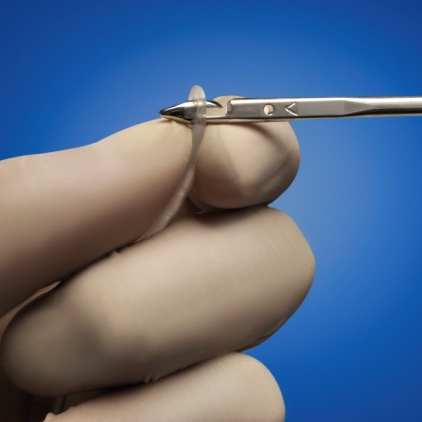
Retropubic Needle
The hooked needle tips are designed to connect to the orifice of the implant's silicone arms for positioning during surgery.
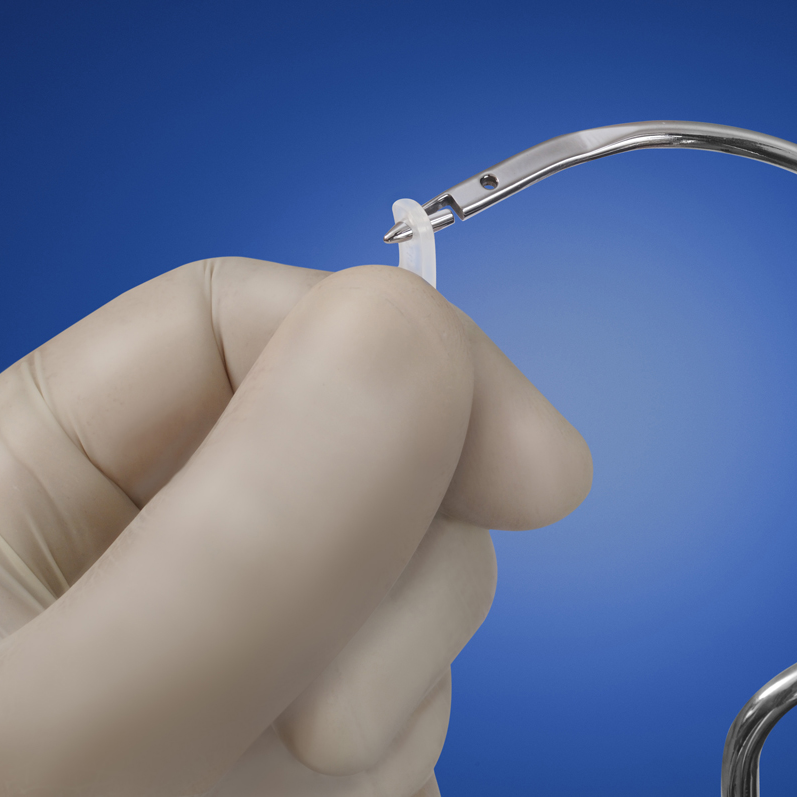
Transobturator Needle
SURGICAL TECHNIQUE
CONTRAINDICATIONS &
PRECAUTIONS
The main contraindications for the implantation of ARGUS are:
- Patients with untreated urinary tract infections.
- Patients on anticoagulant therapy.
- Patients with urinary tract obstruction.
- Patients with renal insufficiency.
- Patients with autoimmune diseases affecting connective tissues.
EMEA / APAC
| PRODUCT DESCRIPTION* | APPROACH | ORDERING CODE |
|---|---|---|
| ARGUS T |
Transobturator | KIT-MT-01 |
| ARGUS | Retropubic | KIT-M-01b |
LATAM
| PRODUCT DESCRIPTION* | APPROACH | ORDERING CODE |
|---|---|---|
| ARGUS T |
Transobturator | KIT-MT-01 |
| ARGUS | Retropubic | KIT-M-01b |


