
PRODUCT INSIGHTS
TREATMENT FOR RECURRENT & COMPLEX ANTERIOR PELVIC ORGAN PROLAPSE
Calistar S is intended for the transvaginal reestablishment and reinforcement of the physiological anatomy of the pelvic floor. It is intended for non-fertile1 women with anterior pelvic organ prolapse (POP) with or without involvement of the apical vaginal wall, in both recurrent prolapse or primary POP when other surgical procedures are expected to fail1.
EFFECTIVENESS AND SAFETY OF ANTERIOR POP REPAIR VIA TRANSVAGINAL ROUTE
A total of 107 non-fertile women who underwent transvaginal POP repair with Calistar S for either recurrent (87%) or complex (13%) anterior POP with a mean follow-up of 19 months were enrolled in a multicenter cohort trial:
- The average time for Calistar S implantation was only 38 Minutes
- In 98% the Treatment was successful, defined by POP-Q ≤ 1
- The Exposure rates were very low with 5.6%
In conclusion, Calistar S can be considered effective and safe in women with recurrent or complex anterior POP2.

- Calistar S Instructions for Use 2020.
- Marschke J, Tunn R, Mörgeli C, Kolterer A, Naumann G. Ultra-lightweight mesh Calistar S for transvaginal mesh repair in recurrent and complex anterior pelvic organ prolapse: a multicenter cohort study. 45th IGUA Meeting – Virtual 2020.
PRODUCT HIGHLIGHTS
ULTRA-LIGHTWEIGHT MESH
The Calistar S implant is composed of biocompatible type 1 macroporous, monofilamentous polypropylene with two anterior attachment arms and two posterior mesh arms. The reliable four-point fixation resists a pull-out force exceeding up to four times the maximum abdominal pressure.
I’m interactive - move and click me!
Too small? Please click here
TISSUE ANCHORING SYSTEM TAS
The TAS was developed to provide a reliable fixation to the sacrospinous ligament (SSL). The TAS is composed of a polypropylene anchor with an attached polypropylene suture. With its six circumferentially arranged polypropylene spikes and the safety stop at the bottom of the anchor, the TAS provides:
- High pull-out force and accuracy
- Reliable fixation
- Safety with regard to vascular and neural structures, due to the additional protection tube provided for the implantation procedure

RETRACTABLE INSERTION GUIDE – RIG
The Retractable Insertion Guides were developed to reach the targeted areas for performing an accurate and safe anchor insertion. With their ergonomic design, small diameter and retractable mechanism for connecting and releasing the anchors, both RIGs provide:
- Precision and safety in surgical maneuvers
- Minimal dissection requirement
- Total control of connection and release of anchors
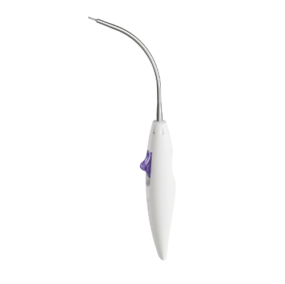
The small RIG was developed to place the anterior attachment arms of the implant into the internal obturator muscle. Its curved design facilitates the introduction and placement of the anchor in the correct angle.
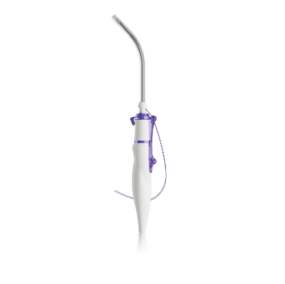
The configuration of the large RIG , with its protective tube, guarantees the integrity of surrounding tissue during implantation of the TAS into the sacrospinous ligament
ANTERIOR ANCHOR RIG
TAS RIG
SURGICAL TECHNIQUE
CALISTAR S IN DAILY CLINICAL PRACTICE:
- Recurrent anterior compartement prolapse with or without involvement of the apical vaginal wall
- Complex primary anterior compartment prolapse with or without involvement of the apical vaginal wall
SURGICAL TECHNIQUE
CALISTAR S IN DAILY CLINICAL PRACTICE
- Recurrent anterior compartment prolapse with or without involvement of the apical vaginal wall
- Complex primary anterior compartment prolapse with or without involvement of the apical vaginal wall
CONTRAINDICATIONS &
PRECAUTIONS
Calistar S must not Be used in:
- Fertile women
- Patients with any active or latent infection of the vagina, cervix or uterus
- Patients with previous or current vaginal, cervical or uterine cancer
- Previous, current or planned pelvic radiation therapy
- Known allergy to polypropylene
The implantation of Calistar S should be based on a thorough patient assessment along with the patient’s individual characteristics and preferences. The following items must also be considered:
- Calistar S must ONLY be used by surgeons experienced in transvaginal pelvic floor reconstruction
- The surgical technique brochure must be read and understood PRIOR to the first implantation of Calistar S.
For further precautions and warnings, we refer to our Instruction for Use.






