PRODUCT INSIGHTS
SINGLE INCISION APICAL REPAIR
SPLENTIS is a single incision repair system, indicated for transvaginal bilateral sacrospinous cervicopexy of the apical pelvic organ prolapse in non-fertile women1. The sling alike implant is fixated bilaterally to the sacrospinous ligaments and centrally to the anterior supravaginal portion of the cervix. This implant configuration uses the minimal amount of alloplastic material, does not interfere with the anterior vaginal wall and demonstrates therefore excellent treatment success while minimizing adverse events2,3.
ADVANTAGOUS ANATOMICAL RESULTS
Splentis provides uterine suspension by bridging the distance between the cervix and the sacrospinous ligaments bilaterally. The apical fixation provides a physiologic, central position while preserving uterine mobility. It proved excellent clinical outcome by simultaneously minimizing the advent of adverse events by avoiding direct contact to the vaginal wall2.
EFFECTIVENESS AND SAFETY OF APICAL POP REPAIR VIA TRANSVAGINAL ROUTE
A total of 103 non-fertile women with a median age of 68 years who underwent transvaginal POP repair with Splentis for primary apical POP were enrolled in a monocentric cohort trial. The median time of surgery was 22 minutes and there were no intraoperative complications, particularly no cases of injury to surrounding vessels or organs. An additional anterior colporrhaphy was performed in 102 (99%) patients. After a median follow-up of 17 months the absence of a vaginal bulge symptom was reported by 91 (89.2%) patients and no patient required repeat surgery due to prolapse recurrence, indicating a treatment success in 91 patients. Subjectively, a total of 99 (97.1%) patients reported treatment success. Exposure occurred in three patients (2.9 %), whereas further surgery was mandatory in two patients (1.9%) and one patient (1.4%) presented complete remission by conservative treatment. In conclusion, Splentis can be considered effective and safe in non-fertile women with apical POP3.
- Splentis Instructions for Use 2024.
- Naumann, G. et al.: Positive effects of vaginal bilateral hysteropexy with Splentis-tape on prolapse symptoms and quality of life in women with severe pelvic organ prolapse; Geburtshilfe Frauenheilkd 2018, 78(10):219-220.
- Naumann, G. et al.: A novel bilateral anterior sacrospinous hysteropexy technique for apical pelvic organ prolapse repair via the vaginal route: a cohort study. Arch Gynecol Obstet (2022). https://doi.org/10.1007/s00404-022-06486-4
PRODUCT HIGHLIGHTS
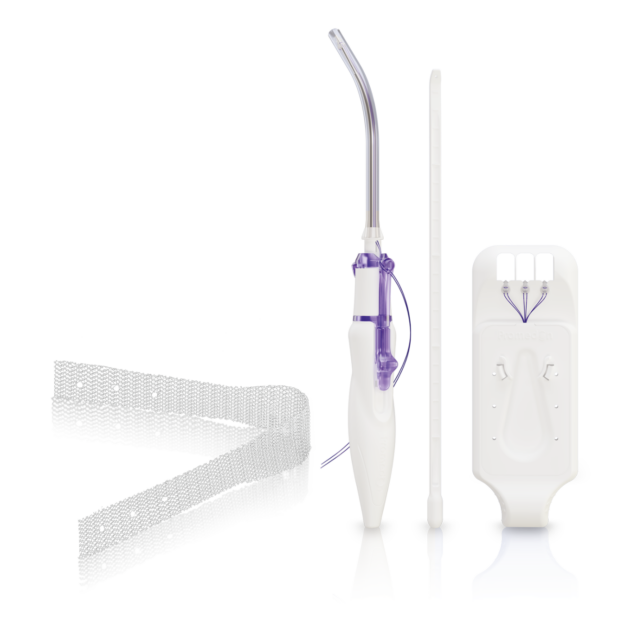
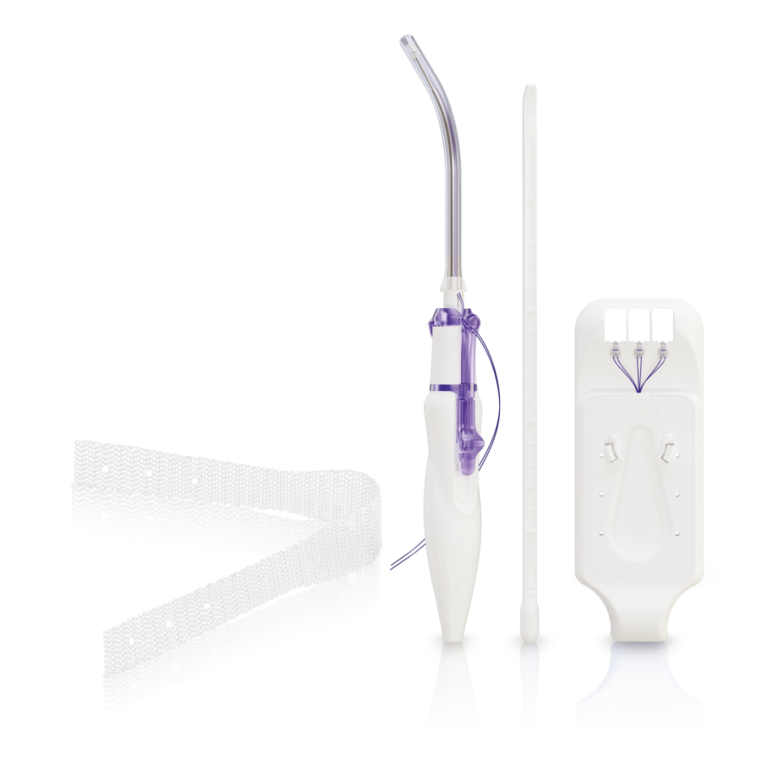
SPLENTIS REINFORCING IMPLANT
Splentis is composed of biocompatible, lightweight, type 1 (macroporous, monofilamentous) polypropylene. The sling alike configuration of Splentis uses the lowest amount of alloplastic material to achieve a reliable physiological apical suspension in a central position.
I’m interactive - move and click me!
Too small? Please click here
TISSUE ANCHORING SYSTEM TAS
The TAS was developed to provide a reliable fixation to the sacrospinous ligament (SSL). The TAS is composed of a polypropylene anchor with an attached polypropylene suture. With its six circumferentially arranged polypropylene spikes and the safety stop at the bottom of the anchor,
the TAS provides:
- High pull-out force and accuracy
- Reliable fixation
- Safety with regard to vascular and neural structures, due to the additional protection tube provided for the implantation procedure

RETRACTABLE INSERTION GUIDE – RIG
The Retractable Insertion Guide was developed to reach the targeted area for performing an accurate and safe anchor insertion. The configuration of the RIG, with its protective tube, guarantees the integrity of surrounding tissue during the implantation of the TAS into the sacrospinous ligament. With its ergonomic design, small diameter and retractable mechanism for connecting and releasing the anchors, the RIG provides:
- Precision and safety in surgical maneuvers
- Minimal dissection requirement
- Total control of connection and release of TAS anchors
SURGICAL TECHNIQUE
SURGICAL TECHNIQUE
SPLENTIS IN DAILY CLINICAL PRACTICE
- Descent of the uterus
- Descent of the uterine cervix after subtotal (supracervical) hysterectomy
CONTRAINDICATIONS &
PRECAUTIONS
Splentis must not be used in:
- Fertile women
- Women with post-(total) hysterectomy vaginal vault prolapse
- Patients with any active or latent infection of the vagina, cervix or uterus
- Patients with previous or current vaginal, cervical or uterine cancer
- Previous, current or planned pelvic radiation therapy
- Known allergy to polypropylene
The implantation of Splentis should be based on a thorough patient assessment along with the patient’s individual characteristics and preferences. The following items must also be considered:
- Splentis must ONLY be used by surgeons experienced in transvaginal pelvic floor reconstruction
- The surgical technique brochure must be read and understood PRIOR to the first implantation of Splentis
For further precautions and warnings, we refer to our Instruction for Use.
EMEA / APAC
LATAM
ORDERING INFORMATION
ORDER NUMBER: KIT-UT-01



